Each bond uses two valence electrons. If you arent sure about co-ordinate dative covalent bonding you arent going to make much sense of what follows without first following this linkAmongst other examples of co-ordinate bonding that page contains a description of the bonding in the complex ion formed between aluminium ions and water molecules and that will be repeated below on this page - so.

Rules For Lewis Structures Pre Ap Adapted From Brown And Lemay Ppt Download
How to Identify the Molecules Having Resonance.
. NO 3 d. Usually single-atom with the least electronegativity becomes the central atom. Determine the shape of the molecule.
398 For example in the molecule methyl isocyanate H 3 C-NCO the two carbons and one nitrogen are central atoms and the three hydrogens and one oxygen are terminal atoms. A central atom is defined in this theory as an atom which is bonded to two or more other atoms while a terminal atom is bonded to only one other atom. Resonance exists only when a Lewis structure has multiple bonds and an adjacent atom with at least one lone pair.
The electronic configuration of carbon is 1s2 2s2 2p2 as its atomic number is 6. For the following molecules or ions where the central atom is underlined. Count the total number of valence electrons in the molecule or polyatomic ion.
Drawing the Lewis Structure for CO. The Lewis structure for CO has 10 valence electrons. If it is not neutral add an electron for.
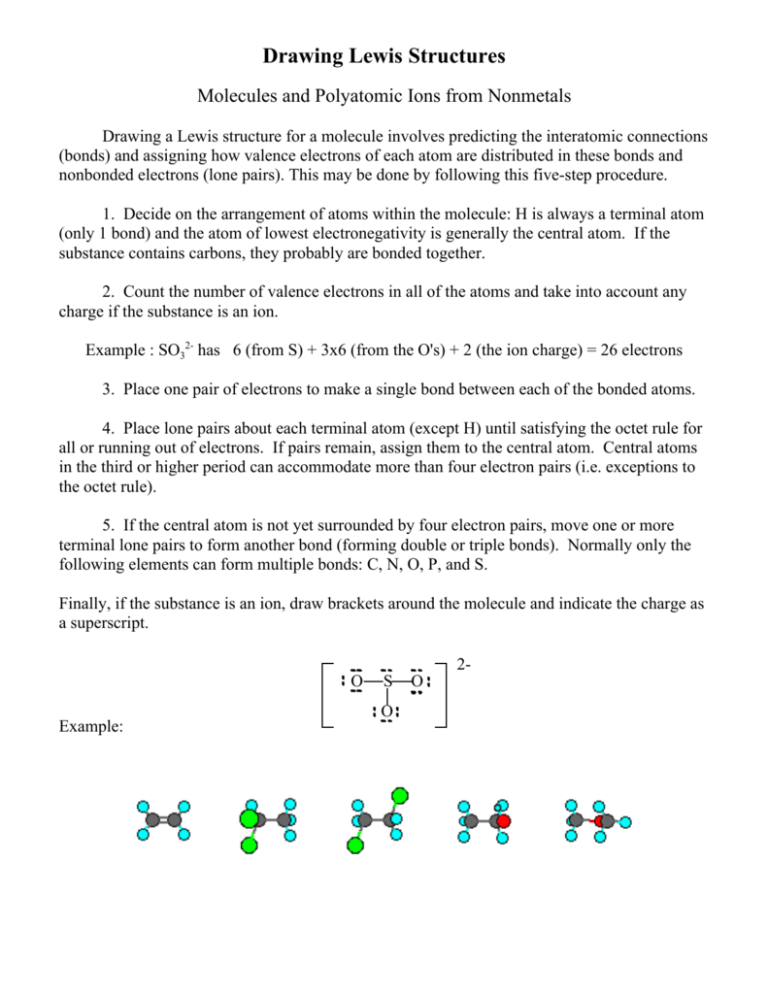
Once we know how many valence electrons there are in PO4 3- we can distribute them around the central atom with the goal of filling the outer shells of each atom. Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO 3 2-ion. Now let us study the step-by-step method for drawing the Lewis structure of Chloromethane CH3Cl.
PO 4 3 b. H is NEVER UNDER ANY CIRCUMSTANCES a central atom. As the Carbon C.
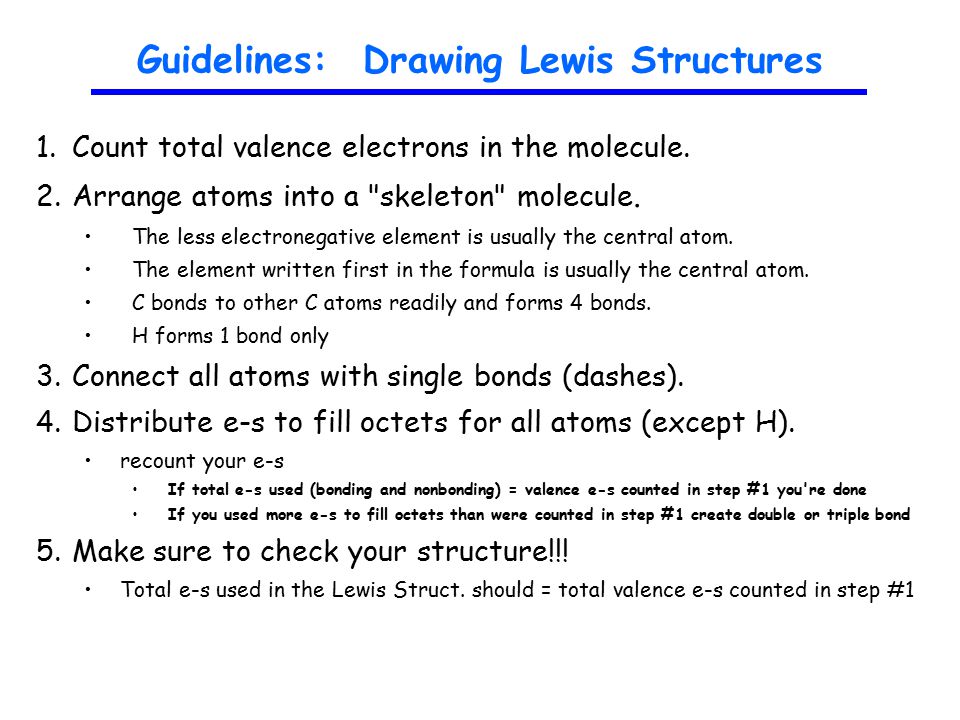
By In both Lewis structures there are eight lone pairs on all oxygen atoms. Combining atoms to form covalent molecules can be accomplished following five easy steps. Sometimes there are several correct Lewis structures for a given molecule.
CH 2 Br 2 d. NH 4 c. Draw the Lewis dot structures for each of the following molecules.
Drawing the Lewis Structure for CO. H 2 S c. 416 The geometry of the central atoms and.
The first step of drawing resonance structures starts with drawing all the possible Lewis structures. 1 Find the total number of valence electrons for a neutral molecule by adding up the number of valence electrons needed for each atom in the molecule. Distribute the remaining valence electrons in pairs so that each atom obtains eight electrons.
The general form of. After determining how many valence electrons there are in CO place them around the central atom to complete the octets. Rules for Drawing Lewis Structures Complete the Lewis structures in the table on the datasheet.
Therefore five electron groups are around the central atom of. How many electron groups are around the central atom. Draw a single bond from each terminal atom to the central atom.
Drawing the Lewis Structure for PO 4 3- For the PO4 3- Lewis structure use the periodic table to find the total number of valence electrons for the PO4 3- molecule. Central atom of SO 3 2-ion is sulfur. Rules for Writing Lewis Structures.
In the case of CH3Cl there are only two single atoms C and Cl where their electronegativity values are 26 and 32. To begin with understanding the Lewis structure of methylamine CH3NH2 it is first crucial to understand how many valence electrons each participating atom has. For the CO Lewis structure calculate the total number of valence electrons for the CO molecule.
Draw the Lewis dot structure for each of the following polyatomic ions. Draw the Electron dot structure. The compound is a chain of three oxygen atoms and minimizing the charges while giving each atom an octet of electrons requires that the central oxygen atom form a single bond with one terminal oxygen and a double bond with the other terminal oxygen.
Ozone O 3 O_3 O 3 is one example. When more than one Lewis diagram can be drawn for a molecule the diagrams are called resonance structures. Find the central atom.
If it has only one Lewis structure it doesnt have a resonance hybrid. CO 3 2 4.
Guidelines Drawing Lewis Structures Ppt Video Online Download

Step 1 Identify The Central Atom And Draw It S Lewis Structure This Is The Element With The Lowest Number Of Atoms In The Formula Draw A Lewis Dot Diagram Ppt Download

Lewis Structures Multiple Central Atoms Science Chemistry Chemical Bonds Showme

Aleks Writing Lewis Structures For A Molecule With One Central Atom And No Octet Rule Exceptions Youtube

How To Draw Lewis Structures For A Molecule With One Central Atom No Octet Rule Exceptions Chemistry Study Com
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)

0 comments
Post a Comment